
Document Management that Guarantees Protection of Companies and Ensures the Quality of Products and Services
In recent years, there have been many cases of data falsification and other such improper management of documents/records in the automobile and construction industries, which has seriously damaged the brand image of some companies. Mr. Jun Takahashi of Nomura Research Institute, Ltd. (NRI), who has many years of experience with document management systems, spoke to us about the issues with and importance of managing documents/records, including the difficulties in establishing an efficient yet safe and secure method.
Why is it difficult to manage documents/records appropriately?
Conventional document management systems are primarily intended to store files and are not equipped to deal with data falsification. In the case of electronic data, they are not reliable as they can be overwritten and deleted. For this reason, while documents/records are managed in electronic format, it is common to also store the same content as an original paper document by adding a signature and seal. However, this still leaves room for tampering by replacement of documents/records or electronic data.
In addition, Japanese companies in the past achieved growth largely due to the technical skills and sales capabilities of frontline employees. As a result, the frontline offices were given great discretionary power and entrusted with the management of important documents/records. Another factor behind their effective functioning was their “self-sufficiency”, which kept all operations in-house, and low employee turnover, which meant that everyone could be trusted.
However, the corporate environment and business models are changing significantly. Business processes are increasingly becoming complex in many companies, and it is common to observe things like a large number of employees involved in a single product or service or outsourcing of part of the work. Business frameworks, such as the horizontal and vertical division of labor in the value chain and the optimization of global allocation, have changed drastically and are now based on a variety of collaborations, making it essential to digitalize and centrally manage documents/records. For example, the sharing of design documents with partner companies in the manufacturing industry or sharing of information on design, construction, and management of buildings and apartments with the construction industry, requires centralized management that goes beyond the boundaries of companies.
As the number of stakeholders continues to grow, the conventional approach to document management that was driven by good nature and trust is reaching its limits.
Necessity of reviewing business processes for rigorous document management
When events such as data falsification or leakage come to light, they impact not only the corporate brand image but also business operations. Considering this, we can say that managing documents/records appropriately is an important management issue. The issues that need to be addressed are establishment of a lifecycle management system for documents/records and visualization of the same using digital technology for future viewing.
This specifically includes: (1) digitalizing documents/records and getting them electronically signed on time by the concerned person to validate it; (2) not mixing valid electronic data with invalid electronic data such as data that is under process or expired; (3) keeping an audit trail of who did what and when, and being able to easily check the operation history; and (4) storing electronic data in such a form that cannot be tampered with over a long period of time.
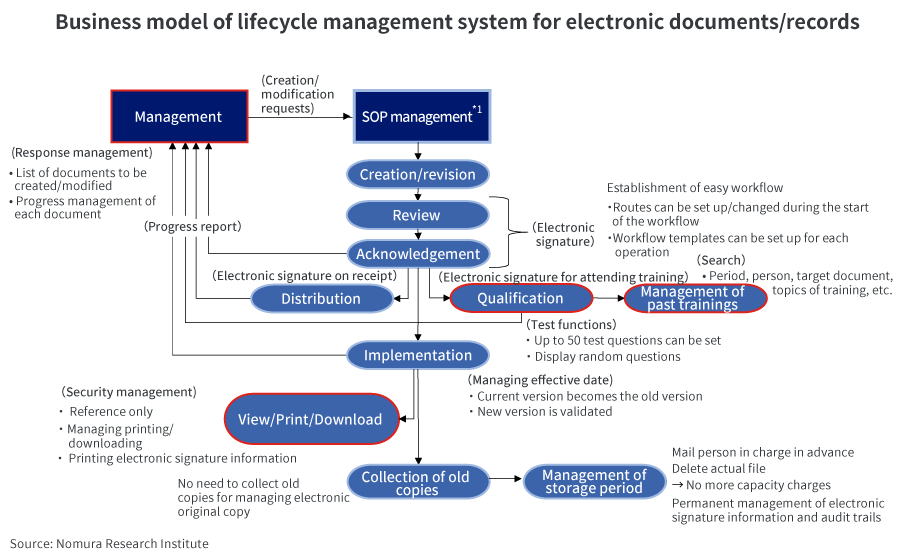
However, until now, such document management systems have been expensive and only used by some companies.
Furthermore, as document management is not directly related to sales and profits, it is not given priority. As a result, even though everyone was aware of the need for safe and secure document management, the associated risks were not well understood by the management.
A leading document management system for the pharmaceutical industry
Since the 1990s, the U.S. FDA *2 and the Ministry of Health, Labor and Welfare (MHLW) have called on the pharmaceutical and medical device industries to manage documents/records related to the quality of their products rigorously, as they are subjected to verification during inspections.
For this reason, pharmaceutical and medical device manufacturers have established a document management system that ensures the reliability of surveys and research data during the period from research and development to market launch, and post that during the period of sales (typically several decades). It is essential to identify the authors and approvers of the documents/records, clarify the validity of such documents/records, and manage them over a long period of time without leaving any scope for falsification.
NRI offers Perma Document, a document management service for the pharmaceutical and medical device industries. It is a service that centrally manages electronic data storage, version information and distribution, training history, and audit trails, and is being used by around 10,000 users across 45 companies. For more than 10 years since the launch of the service, companies have used it to electronically store documents/records subject to industry regulations, and these have also been inspected by the MHLW and the FDA.
Expanding Perma Document to a wide range of industries and businesses
When the service was launched 10 years ago, we explored the possibility of using cloud computing. However, determining that it was too early to store data in the cloud environment from the perspective of information security technology and public awareness at the time, we instead used NRI's data centers as they enjoyed an impressive reputation in the financial industry.
Now, we have constructed Perma Document on the cloud to ensure scalability and expanded our services to reach more clients.
As it is on the cloud space, it is possible to collaborate with other companies while ensuring security. In addition, all systems have been duplicated and triplicated to ensure continuity of business.
As with information security, improvements in the document management system are usually carried out only after an incident occurs. However, the use of a highly reliable document management system not only improves the overall quality of products and services and credibility of the company, but also protects the corporate brand by serving as public proof of the company's activities in case of an emergency.
NRI will continue to promote rigorous document management in more and more industries.

- *1 SOP management: Standard Operating Procedures
- *2 FDA: Food and Drug Administration, USA
Profile
-
Jun Takahashi
* Organization names and job titles may differ from the current version.