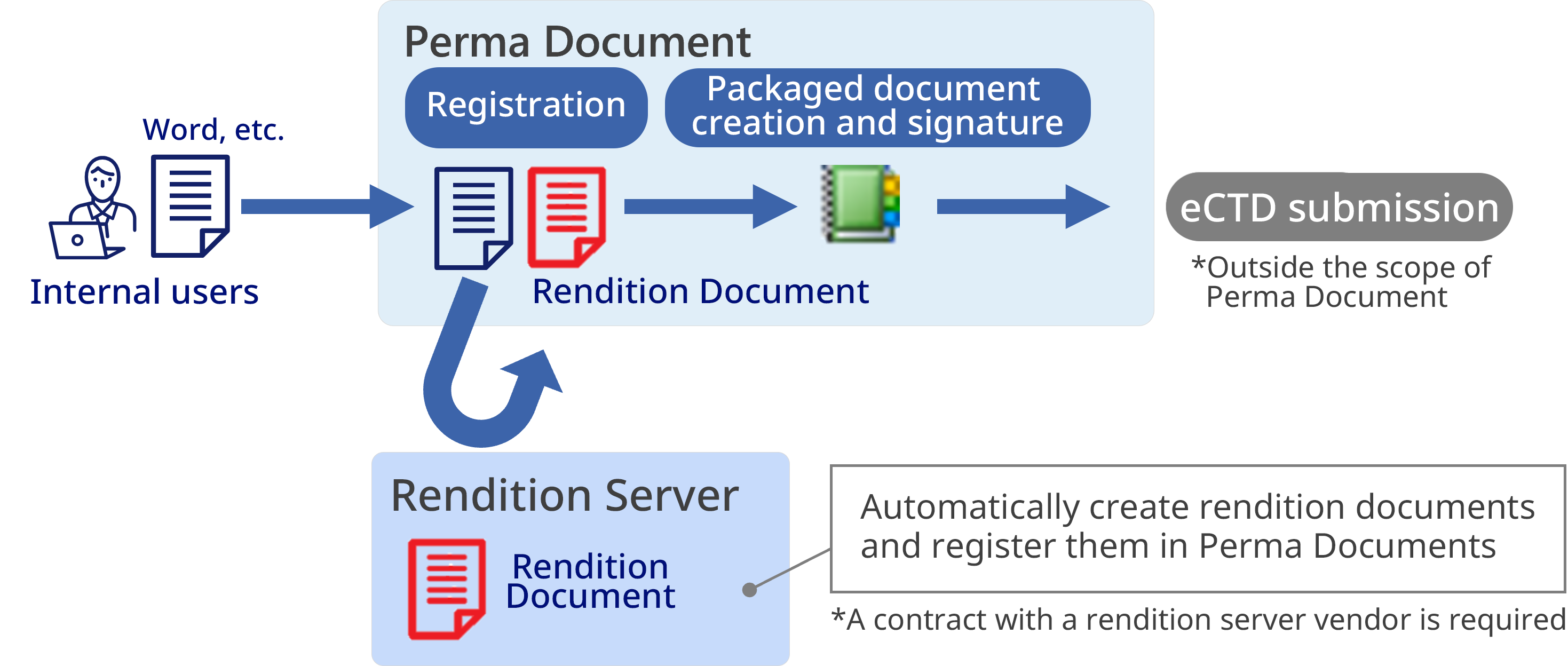
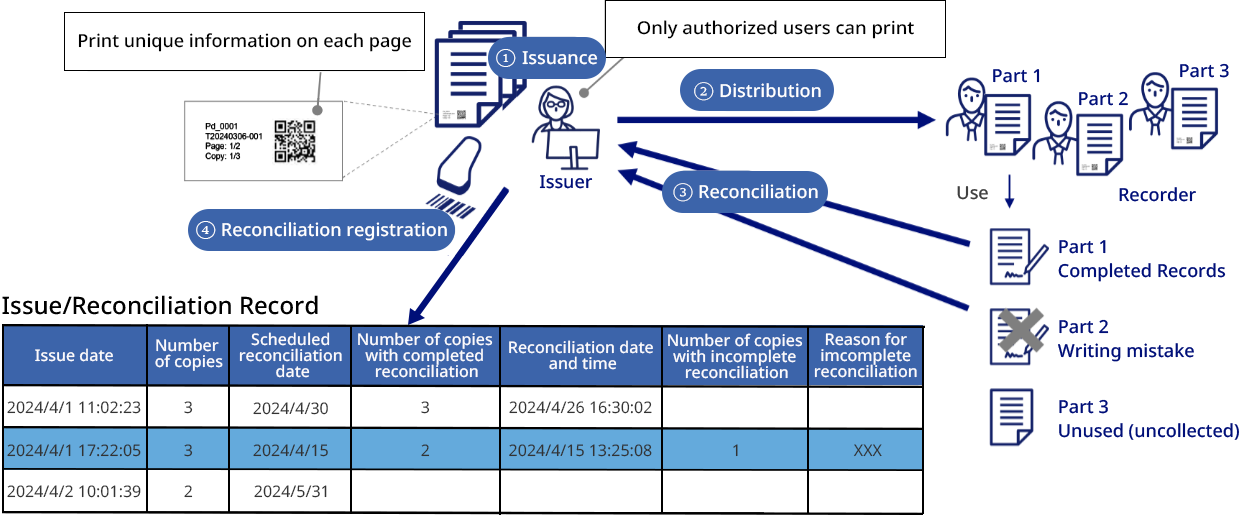
In the research, development, and manufacturing of pharmaceuticals and medical devices, document management is no longer simply a matter of storing files; it is essential to clearly identify the document creator and approver, to clearly indicate whether the document is valid or invalid, and to manage the document for long periods of time in a way that leaves no room for tampering.
Perma Document (GxP Edition) complies with regulations such as ER/ES guidelines and 21 CFR Part 11, and provides the functions required in the pharmaceutical industry. In addition, an option for template documents to support CSV (Computerized System Validation) is also available, allowing for smooth and rapid implementation.
Features of Perma Document
- A document management cloud service that supports ER/ES guidelines, CSV, and data integrity, and does not require the use or creation of a system
- Abundant functions for tasks requiring document/record storage under GxP
- Easy search for relevant documents and records, and flexible response to unannounced inspections
- NRI develops and operates the service domestically, and data is also managed domestically, so users only pay a monthly usage fee
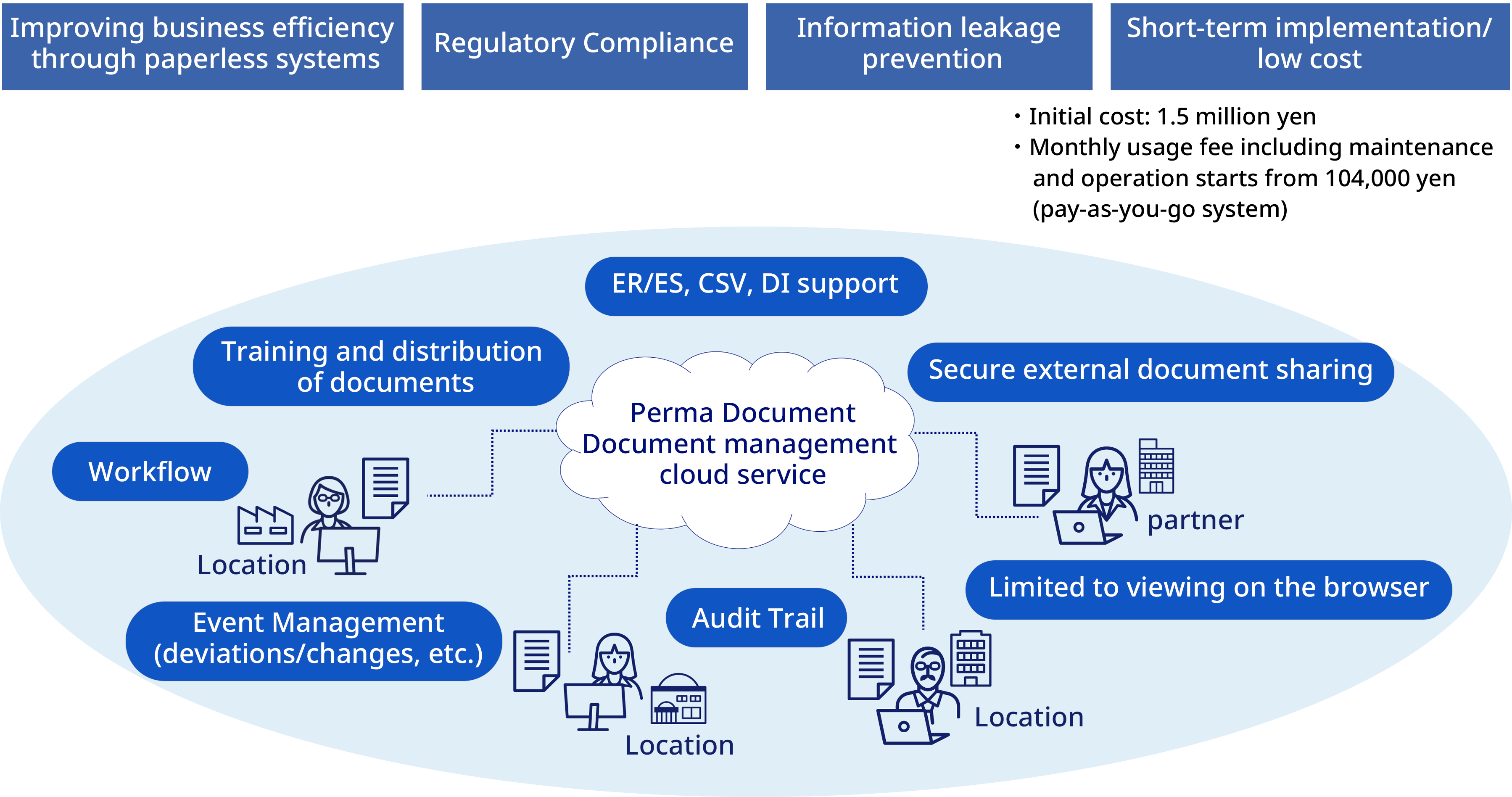
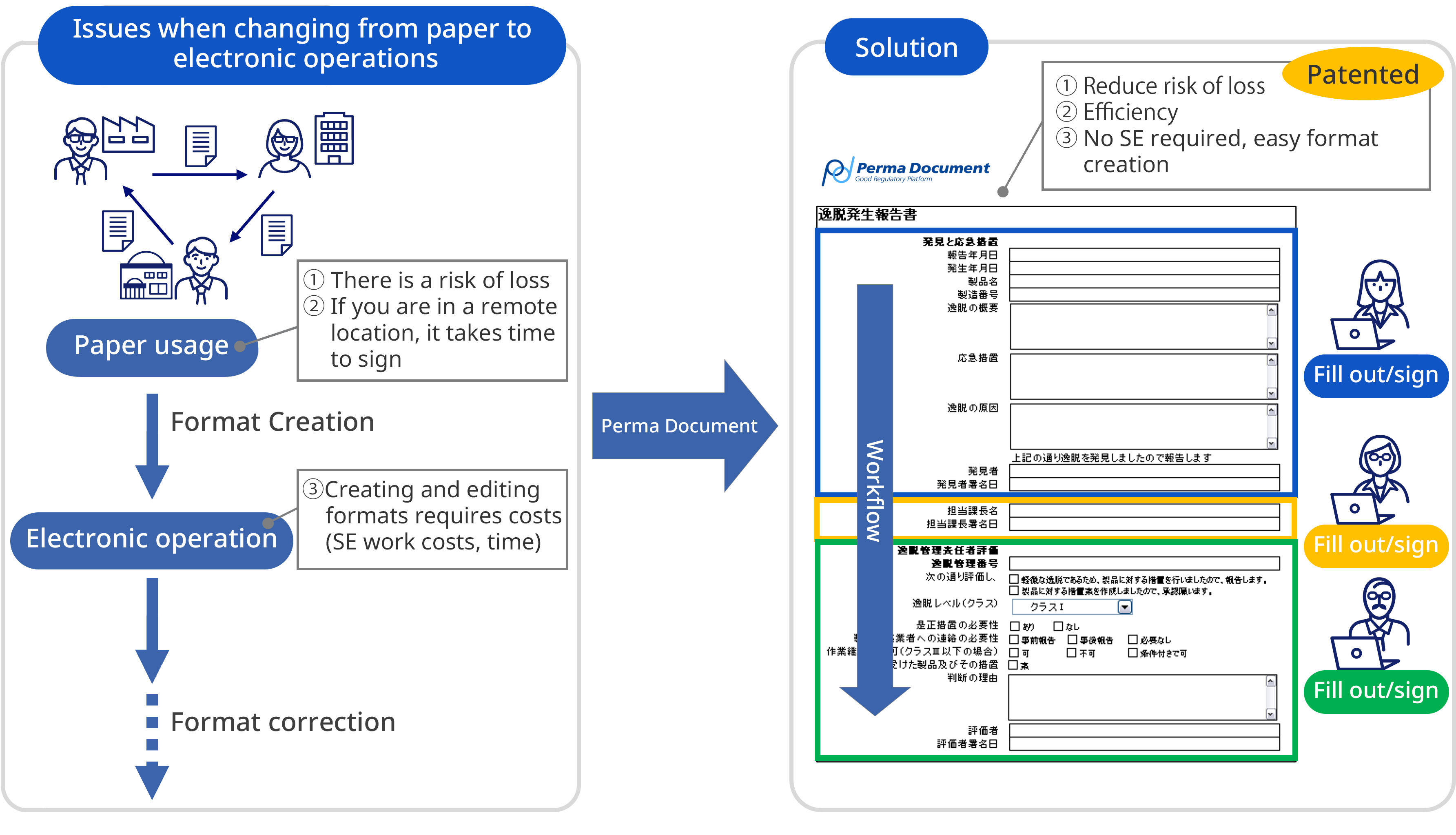
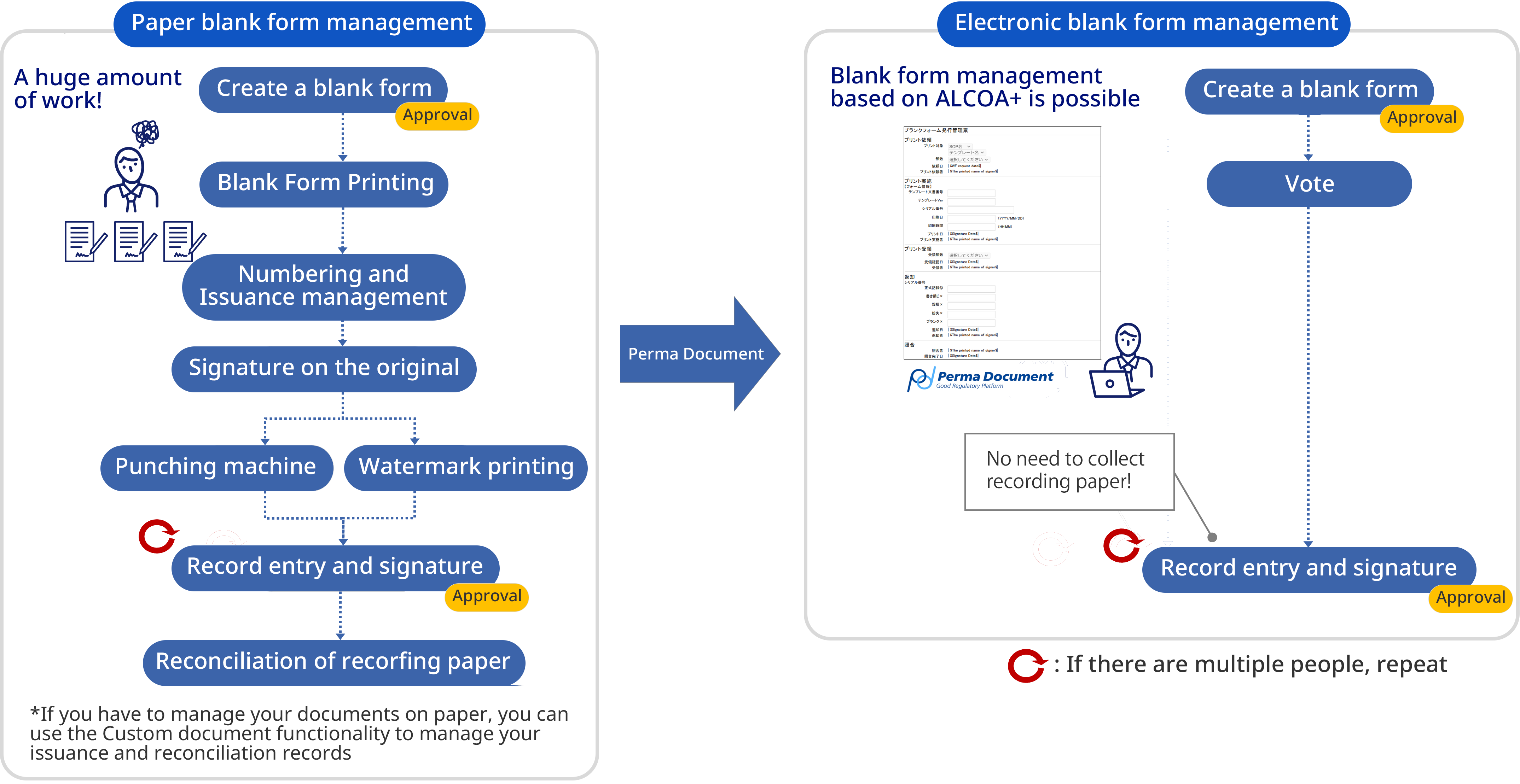
Overall picture of document-related work
- Properly manage the entire document lifecycle, ensuring quality assurance and preventing falsification
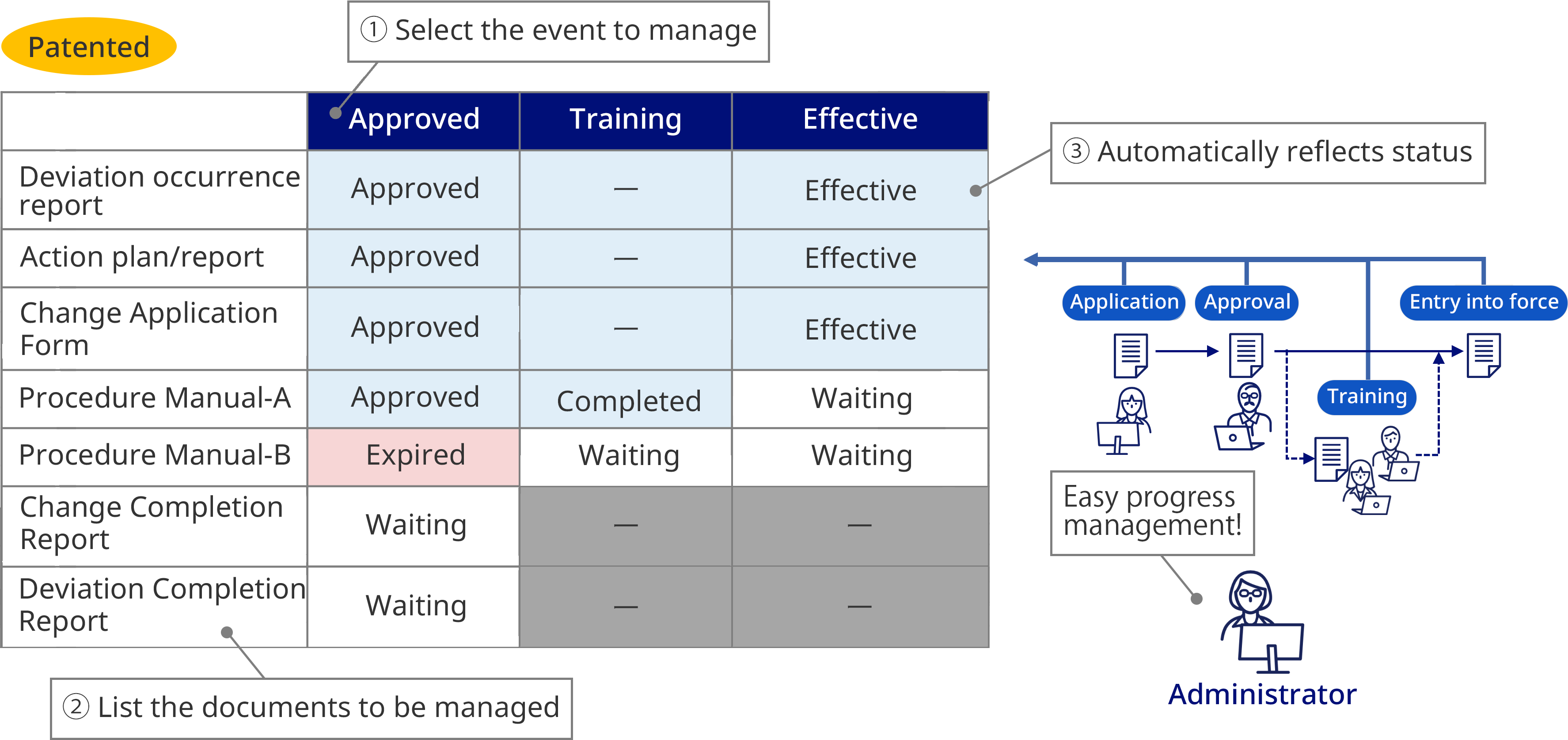
- Easily realize procedure management and progress management (non-conformance, changes, complaint handling, etc.)
- Access control for display, printing, download, etc. for each document is also possible
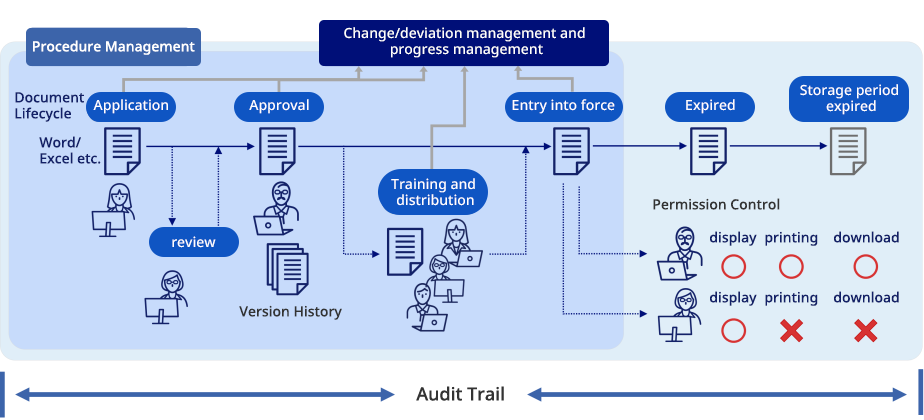
Features List
-
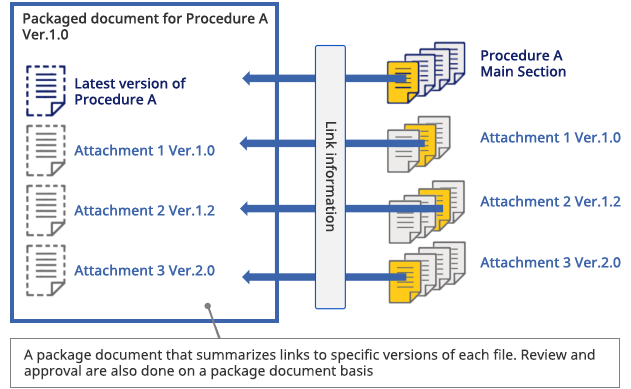
Lifecycle Management 
-
No-overwrite control
Version Control
-
Workflow・
Digital Signature
-
Distribution
Management
-
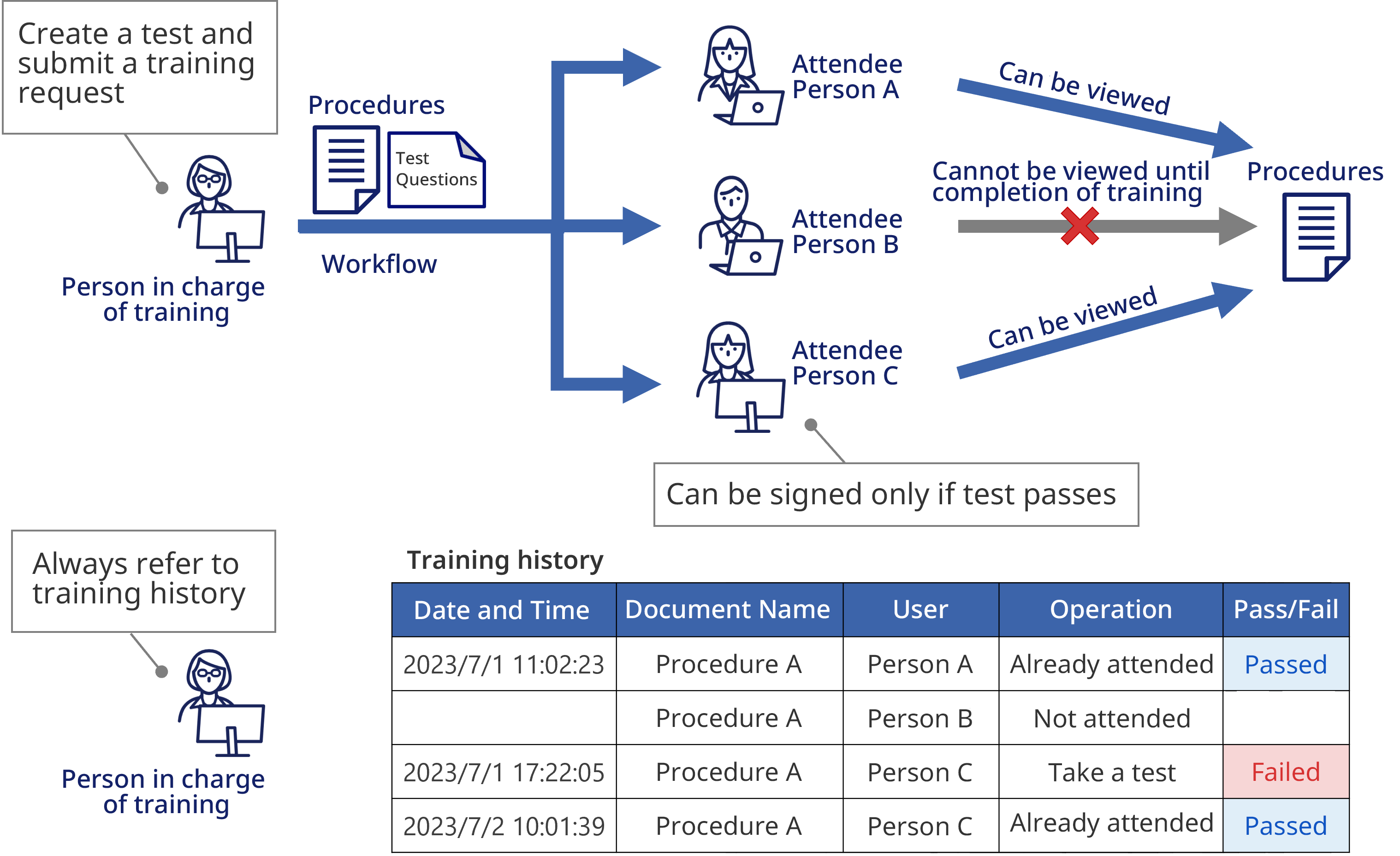
Training history
Management
-
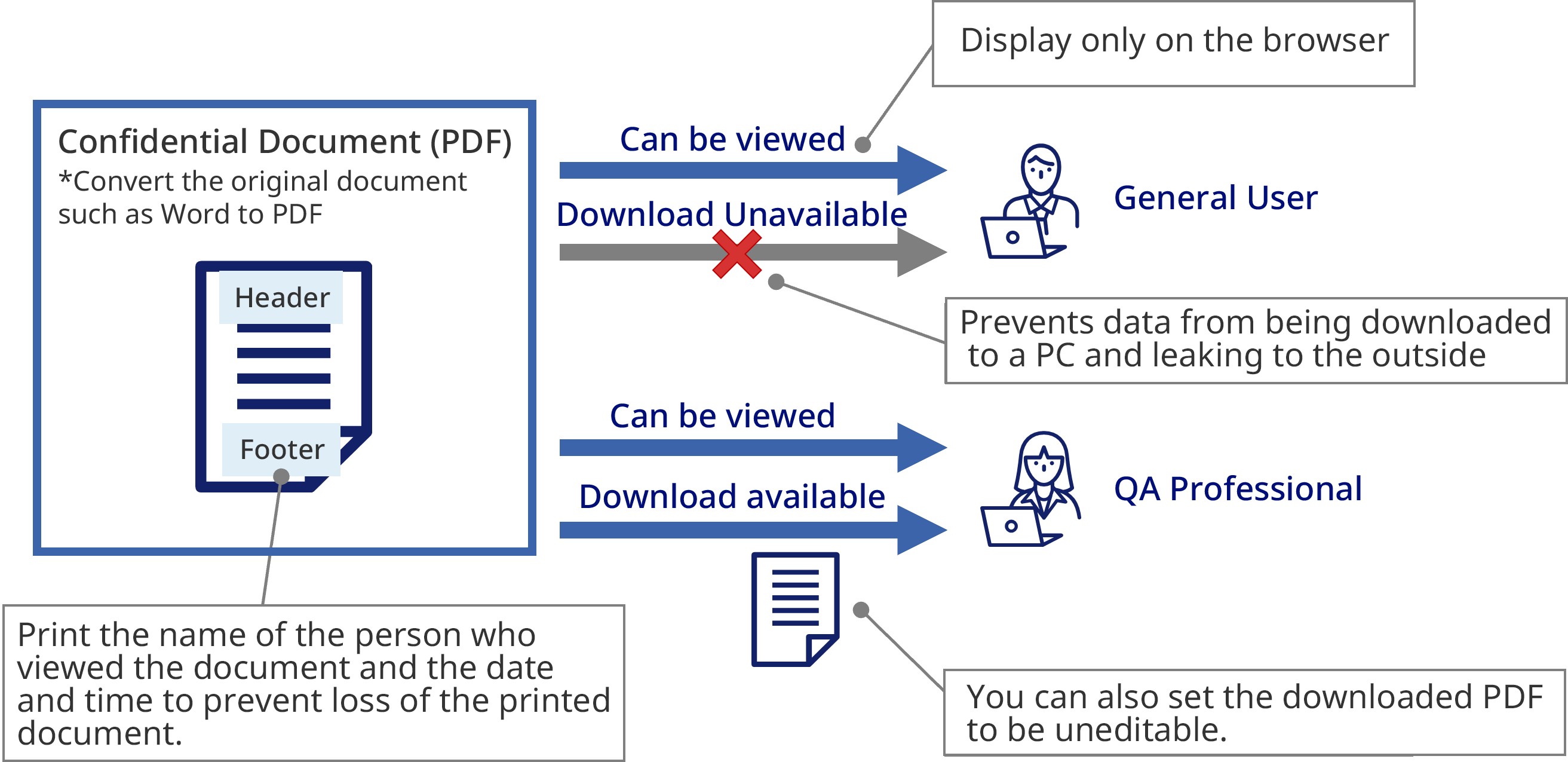
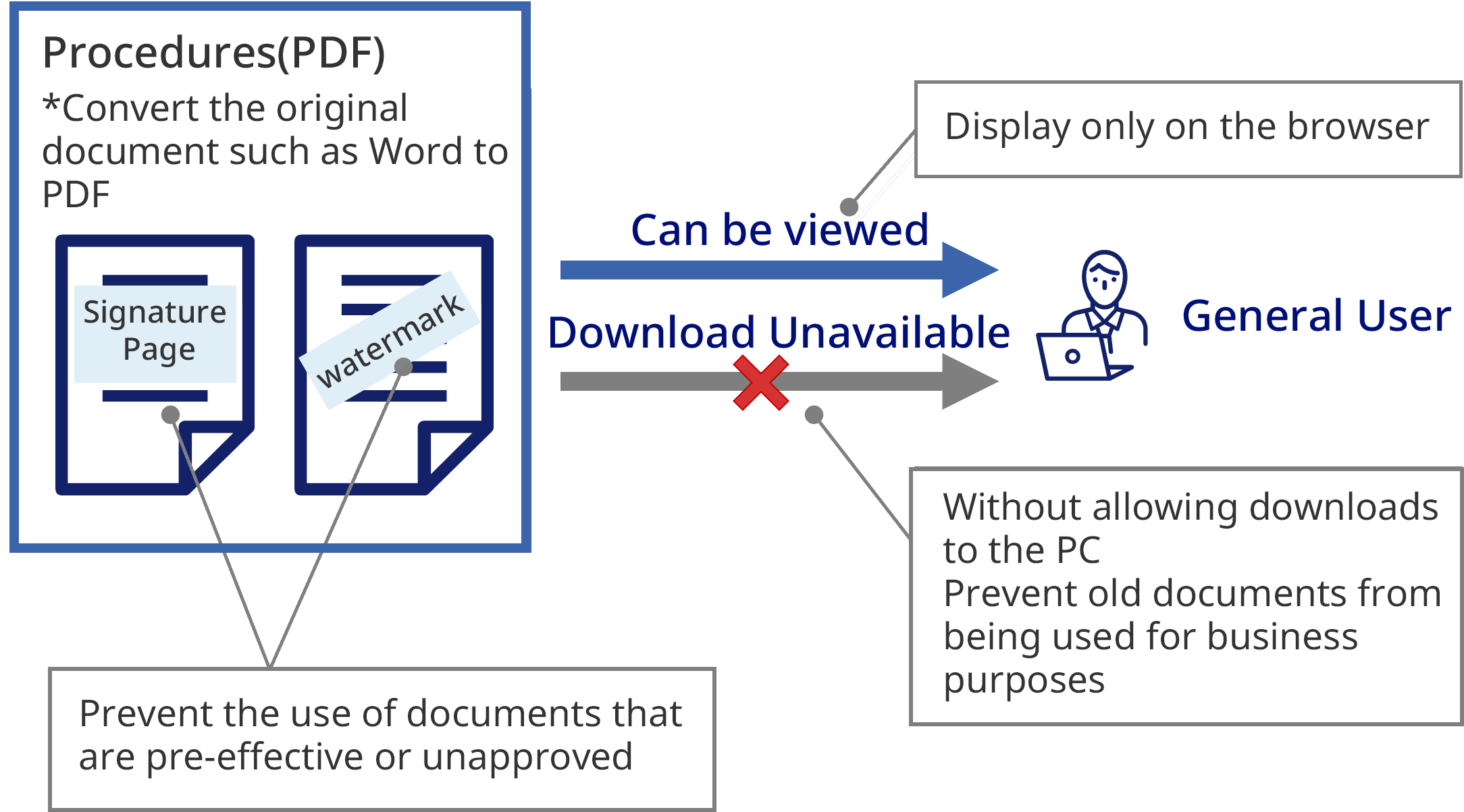
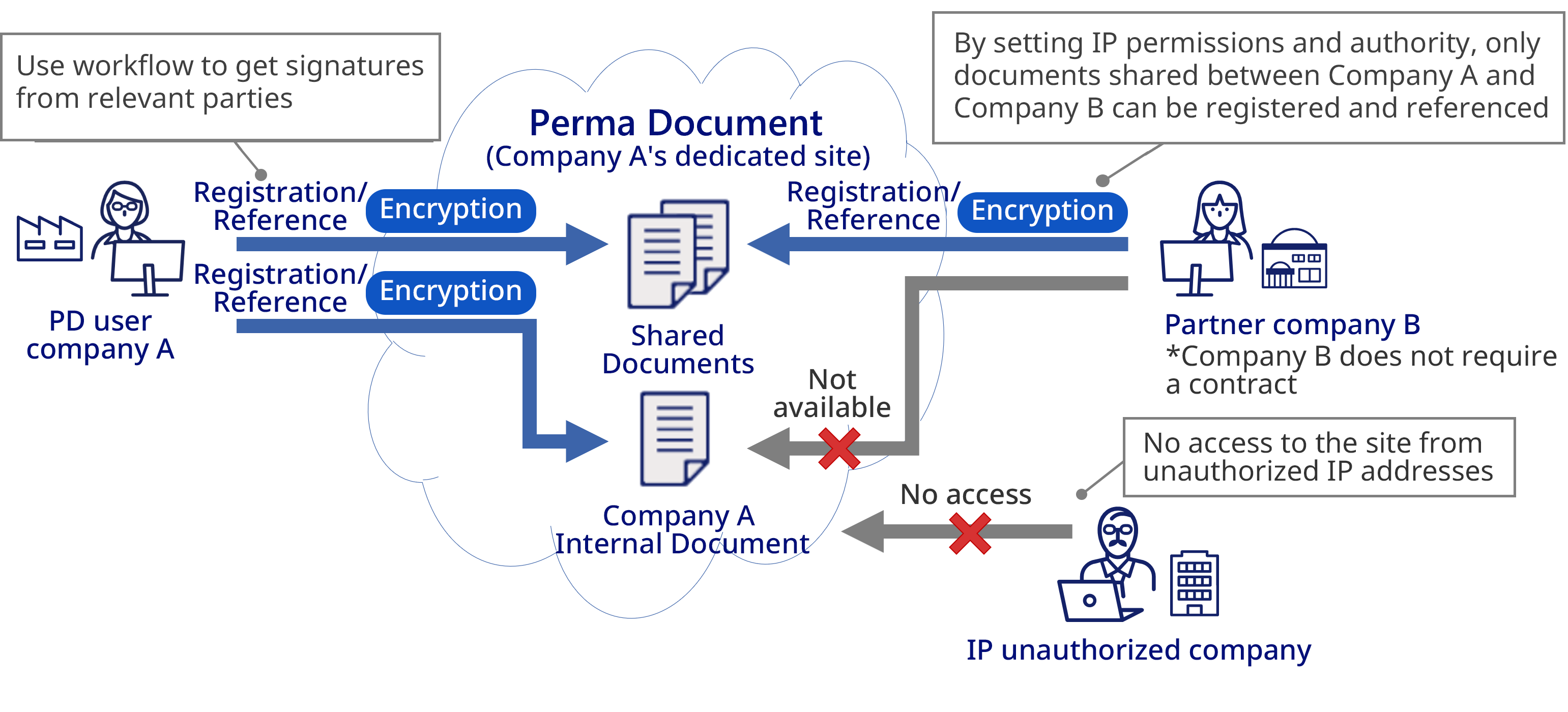
Access Management 
-
Audit Trail 
-
Document Protection 
-
Theme Management・
Custom Document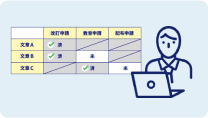
-
Rendition Management 
-
Qualification
Management
-
Traceable Print 
Usage scenarios by issue
It can solve various issues in document management work.
- You can use it in the following situations:
Usage fee
The following fee structure is available: We will provide you with a separate quote for details.
| Initial cost | From 1.5 million yen |
|---|---|
| Monthly fee | From 104,000 yen |
Implementation track record
- Number of companies: 60
- Number of users: 20,000
(As of April 2023)
Operation and maintenance support
- Available 24 hours a day, 365 days a year
- Robust security
- Backup, disaster recovery, and failure recovery
- Help Desk (weekdays 9:00-17:00)
Development Background
In 1997, the U.S. FDA enacted 21 CFR Part 11 to allow electronic records and electronic signatures in connection with new drug applications. Since it was ratified by countries around the world, it has become a de facto global standard. 21 CFR Part 11, as a regulation on electronic records and electronic signatures, is said to have had a major impact on other subsequent legal systems.
| Audit Trail |
|---|
| Security Integrity |
| Computer System Validation |
Then, in the 2000s, domestic pharmaceutical companies also responded to similar regulations and began to move toward computerization. On the other hand, compared to paper documents, proving the integrity of electronic documents (that they have not been tampered with) requires a lot of manual work, creating a new contradiction between efficiency and inefficiency. Furthermore, as the Internet began to be fully utilized and collaboration in the development process of pharmaceutical companies progressed, preventing the falsification of electronic documents became a new challenge when using the Internet.
In response to these requests from pharmaceutical companies, we decided to offer a cloud-based service, which was beginning to gain recognition within the industry at the time. In 2008, we launched our pioneering cloud-based electronic document management service "Perma Document."
NRI has been involved in electronic document management for pharmaceutical companies for over 15 years and has established the ideal form for GxP document management operations.
- Other industries that require quality assurance
- Standard Edition